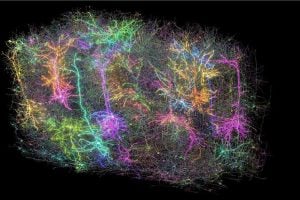
Researchers studying how nerve cells in the brain communicate have created the world’s most comprehensive map of a mouse brain, shedding light on its visual-processing center and how it makes sense of what is seen by the eyes. The interdisciplinary MICrONS Consortium, which includes a Johns Hopkins Applied Physics Laboratory (APL) team led by Brock Wester, vice chair of the Whiting School of Engineering’s Engineering for Professionals Applied Biomedical Engineering program, mapped this region with remarkable precision, down to individual synapses—the physical connections between each neuron in the brain.
The Consortium’s results appear in a package of eight papers published in Nature and Nature Methods, outlining the major contributions from the Intelligence Advanced Research Projects Agency’s nine-year Machine Intelligence from Cortical Networks (MICrONS) program. Launched in 2015, the program built on ideas from a three-year internally funded APL initiative aiming to automate the generation of “connectomes,” or brain maps.
“The IARPA MICrONS program was a massive collaboration between teams of experts focusing on different challenges in the data generation pipeline, from animal experiments and imaging to automated image processing, curation, and analysis,” said Brock Wester, who led the APL MICRoNS team. “In addition to developing cloud infrastructure and analysis tools for petabyte-scale datasets, our team focused on a resource-intensive aspect of the pipeline: proofreading and validation methods to make sure the data was accurate and ready for science.”
To create their connectome, members of the MICrONS team from Baylor College of Medicine (BCM) worked in the laboratory with a genetically engineered mouse whose brain neurons lit up when activated. The rodent ran on a treadmill while watching a variety of movie clips—from action-packed scenes in Mad Max and The Matrix to extreme sports such as downhill mountain biking and base jumping—while researchers recorded 76,000 of its neurons firing in real time.
Researchers from the Allen Institute for Brain Science (AIBS) divided a small sample of the mouse’s brain tissue into 28,000 super-thin slices, much thinner than a single human hair, and used multiple electron microscopes to capture detailed images of each slice. Then, utilizing artificial intelligence and scalable data pipelines, team members at Princeton University carefully pieced each image together like a puzzle to trace the path of connections throughout the entire sample.
“These imaging pipelines generate massive, high-resolution datasets that must be extensively processed using AI. But this processing can lead to a variety of errors that need to be corrected to ensure that the brain’s neurons and their synaptic connections are properly reconstructed,” Wester said.
Working together, the MICRoNS teams from APL, Baylor, the Allen Institute, and Princeton University developed smart tools that automate error detection and correction in brain maps that are also scalable to ensure they can analyze and reconfigure the billions of annotations in the dataset. These tools automate tasks that were previously performed by brain experts and involved manual inspection of thousands of images.
“By drastically cutting the time and cost required to correct errors in the data extracted from high resolution images of the brain, these tools are poised to help accelerate the generation of larger and more accurate brain maps, which will further deepen our understanding of the brain’s connectivity and its role in disease,” said Wester.
The result of the MICrONS program was a groundbreaking map of more than 200,000 cells and half a billion synapses that combines form and function in a way never before accomplished.
The work by APL and others on the MICrONS teams continues on the NIH Brain Initiative CONNECTS program through several funded efforts that build on the successes of the MICrONS program.
“The goal is to make a leap toward mapping an entire mouse brain,” Wester said.
In the meantime, experts say that the visual cortex connectome is more than just a map; it represents a new way of understanding how neurons communicate and could offer new insights into the workings of all mammalian brains, including our own.
More coverage of the work can be found in The New York Times.